The healthcare landscape in North America is in a constant state of evolution, driven by technological advancements, regulatory mandates, and, most critically, an unrelenting focus on patient and provider safety. Within this critical nexus, the market for Closed System Transfer Devices (CSTDs) has emerged as a significant and rapidly expanding sector. These specialized drug delivery tools are essential for protecting healthcare workers from exposure to hazardous drugs, primarily chemotherapy agents, during preparation and administration. The North America CSTD market, encompassing the United States and Canada, is not just growing; it is undergoing a profound transformation as CSTDs move from optional safety measures to essential, standard-of-care equipment.
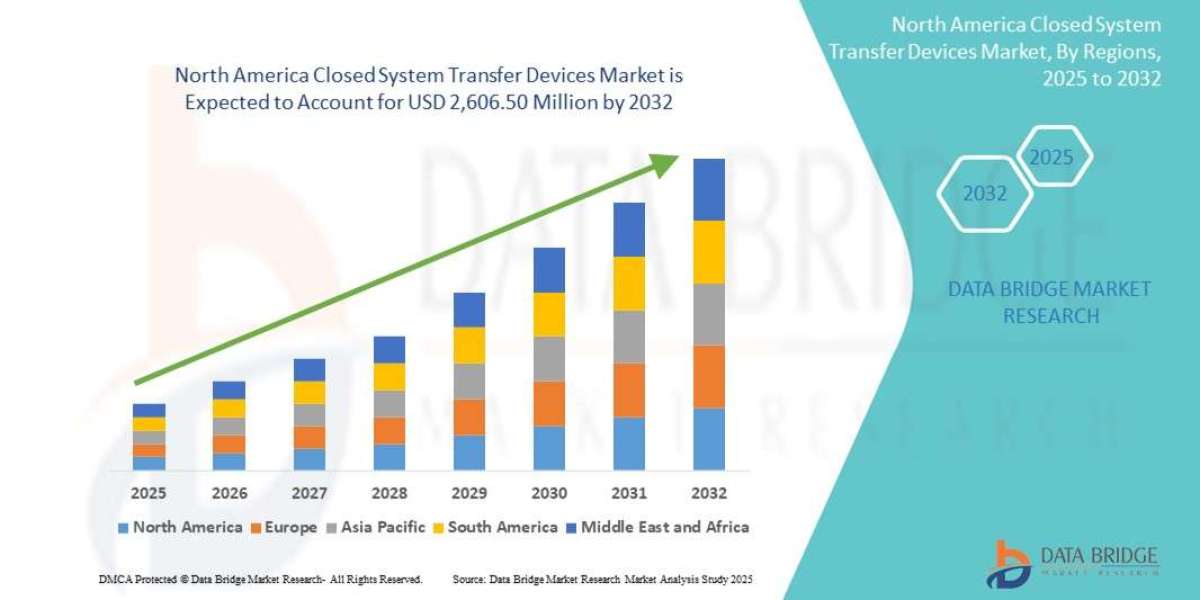
- The North America closed system transfer devices market size was valued at USD 635.23 million in 2024 and is expected to reach USD 2,606.50 million by 2032, at a CAGR of 19.30% during the forecast period
The Imperative: Safety and Regulation
The primary catalyst fueling the North American CSTD market is the stringent regulatory environment and the undeniable clinical need for protection. Hazardous drugs, particularly antineoplastic agents, pose serious health risks to nurses, pharmacists, and technicians, including cancer, reproductive issues, and other long-term toxic effects. This risk has been formally acknowledged and addressed by several influential bodies.
In the United States, the National Institute for Occupational Safety and Health (NIOSH) defined its list of hazardous drugs, creating the framework for safe handling. More influentially, the United States Pharmacopeia (USP) General Chapter $<800>$, “Hazardous Drugs—Handling in Healthcare Settings,” sets enforceable standards for compounding and administering these drugs. This chapter mandates the use of CSTD devices when administering antineoplastic hazardous drugs, if the dosage form allows, and strongly recommends their use during compounding. The enforcement of USP $<800>$ across states has fundamentally altered the purchasing dynamics within hospitals, pharmacies, and clinics, turning CSTD adoption from a choice into a compliance necessity.
Access expert insights and data-driven projections in our detailed North America Closed System Transfer Devices Market study. Download full report: https://www.databridgemarketresearch.com/reports/north-america-closed-system-transfer-devices-market
Canada’s market drivers, while sharing the same commitment to safety, are often guided by provincial-level occupational health and safety regulations and hospital accreditation standards that emphasize safe handling practices. The combined effect of these US and Canadian guidelines has created a robust and non-negotiable demand signal for effective, compliant CSTD technology.
Technological Evolution and Segmentation
The North American CSTD market is highly competitive and characterized by continuous innovation. Devices are typically segmented based on their core mechanism:
Membrane-to-Membrane (or needle-free) Systems: These are the dominant segment. They use components like specialized connectors and elastomeric seals to create a dry, pressure-equalized, and sealed pathway between the drug vial and the administration line. Key players continually refine these designs for better ergonomic use and proven containment efficacy.
Airtight (or mechanical) Systems: These utilize mechanical locking mechanisms, vents with filtering systems, or locking luer connections to maintain a sealed system. While often competing with membrane systems, their differentiation often lies in specialized applications or specific clinical workflow needs.
Beyond the mechanism, the market is segmented by product type (vial access devices, syringe safety devices, bag/line access devices) and end-user (hospitals, oncology centers, clinics). Hospitals remain the largest end-users due to the high volume of chemotherapy preparation and administration services they provide.
The shift in technological focus is moving beyond mere containment to integration and usability. Healthcare providers demand systems that are easy to train on, simple to incorporate into existing workflows, and cost-effective while providing verifiable protection. Compatibility with various drug vials, IV bags, and administration sets is a major factor in purchasing decisions, pushing manufacturers toward standardized yet flexible designs.
Market Dynamics and Future Outlook
The North American CSTD market exhibits several key dynamics:
Consolidation and Competition: The market features a few dominant, established players (often with proprietary technologies) alongside smaller, innovative companies introducing disruptive solutions. Competitive differentiation often revolves around third-party performance studies and clinical data proving zero drug escape or zero microbial ingress, validating the device as truly “closed.”
Increased Home Healthcare Demand: The growing trend of administering chemotherapy and other hazardous drugs in ambulatory and home settings is opening up a new frontier for CSTDs. Devices designed to be intuitive and reliable for use by visiting nurses or even trained patients are becoming increasingly important.
The Total Cost of Ownership: While CSTDs represent a significant initial investment, hospitals increasingly view them through the lens of long-term cost avoidance. The financial and legal risks associated with occupational drug exposure, including potential lawsuits and workers' compensation claims, far outweigh the cost of implementing a comprehensive CSTD program. This economic calculus strongly favors CSTD adoption.
Looking ahead, the market is poised for continued robust growth. Regulatory enforcement will tighten, and USP $<800>$ compliance will become non-negotiable in all jurisdictions. Furthermore, as oncology drug pipelines expand to include more potent and toxic agents, the definition of "hazardous drug" will broaden, thereby expanding the applicability of CSTDs beyond traditional chemotherapy to include certain antivirals, hormones, and immunosuppressants.
Browse More Reports:
Global Liquorice Extracts Market
Global Scientific Instruments Market
Global Sports Optic Market
Global AI in Patient Management Market
Global Digital Transformation of Maritime Freight Market
Global Electronic Weighing Machines Market
Global Cyazofamid Market
Global Residential Lighting Market
Global Spinal Imaging Market
Global Aerial Work Platform Market
Global Biofungicides for Soil Treatment Market
Global Herb Vapourizers Market
Global Plastic Tubes Market
Global Antibiotics in Aquaculture Market
Global Chemical Fungicides Market
The North America Closed System Transfer Devices Market is a compelling example of how safety-driven regulation and technological ingenuity can converge to create a necessary and thriving sector. It is a market whose future success is directly tied to the fundamental ethical imperative of protecting the hands that heal.
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email:- corporatesales@databridgemarketresearch.com