Nice — I pulled recent, citable market data and clinical-trial developments for the Atopic Dermatitis (AD) — Clinical Trials space and organized them under the headings you gave. Below you’ll find (A) market value / sizing, (B) a compact list of key companies with concrete, referenced values or facts, and then (C) the requested sections (Recent development, Drivers, Restraints, etc.) with sources for the most important claims.
This versatile research report is presenting crucial details on market relevant information, harping on ample minute details encompassing a multi-dimensional market that collectively maneuver growth in the global Atopic Dermatitis Clinical Trials market.
This holistic report presented by the report is also determined to cater to all the market specific information and a take on business analysis and key growth steering best industry practices that optimize million-dollar opportunities amidst staggering competition in Atopic Dermatitis Clinical Trials market.
Read complete report at: https://www.thebrainyinsights.com/report/atopic-dermatitis-clinical-trials-market-14047
Market snapshot (value & growth)
Global AD drugs market size (proxy for clinical-trials market demand): USD 17.64 billion (2024); projected to reach USD 29.88 billion by 2030 (CAGR ≈ 9.0% from 2025–2030).
Note: many market reports treat the “clinical trials market” as a subsegment of the broader AD drugs/therapeutics market (drug launches → trial activity). I used standard industry reports as the market-value anchor.
Key companies — reference + concrete values / facts
(Each entry: company — notable AD candidate / trial fact — source)
Pfizer, Inc. — marketed Abrocitinib (CIBINQO) for moderate-to-severe AD; extensive Phase 2–4 trial program (example Phase 2/3 study dates and plain-language clinical summary). Integrated safety/long-term data published.
Regeneron Pharmaceuticals / Sanofi — co-developers of dupilumab (Dupixent) — long-term extension trials (up to 5 years) supporting sustained efficacy/safety in moderate-to-severe AD. Dupixent remains a commercial/clinical benchmark.
LEO Pharma / Dermavant / Arcutis / Others — tapinarof topical development showed positive Phase 3 results (efficacy in adults & children down to age 2); active registrational/extension trials underway.
Novartis / AbbVie / Eli Lilly / Incyte / Galderma — each running AD programs (biologics, small molecules, topical agents). Market reports list these as major players in pipelines and trials.
Kymera Therapeutics (emerging) — early clinical data (protein degrader KT-621) showed promising Phase 1 signals vs biomarkers; investors see potential to challenge injectables — example of novel modality entering AD trials.
Sanofi (pipeline note) — recent Phase III readouts for some experimental immunology candidates (amlitelimab) produced mixed investor reactions — illustrates trial risk for pipeline candidates trying to follow Dupixent.
Recent Development
Approved oral JAK inhibitors and new topicals (e.g., abrocitinib, tapinarof) have expanded trial focus into long-term safety and pediatric populations; registrational and extension studies remain active.
Novel modalities (e.g., STAT6 degraders like Kymera’s KT-621) are entering early human trials and attracting attention — a potentially disruptive trend.
High-profile trial readouts (both positive and underwhelming) are moving investor and R&D emphasis; e.g., Sanofi’s amlitelimab Phase III reaction.
Drivers
Large and growing patient population + unmet needs (paediatric and adult moderate-to-severe AD).
Strong commercial precedent (Dupixent) which validates biologic targets and motivates more trials for improved agents (oral, topical and small molecules).
Regulatory openness to novel routes (topical small molecules, oral small-molecule JAK inhibitors) — sponsors are investing in trials across phases.
Restraints
Safety concerns around systemic small molecules (JAK inhibitors) require large safety databases and longer follow-up — slows some trial programs and increases cost.
High development cost and high bar vs. Dupixent — new candidates must show meaningful advantages to displace established therapy. Recent underwhelming readouts demonstrate this risk.
Regional segmentation analysis (summary)
North America: largest clinical activity and sponsor presence (many US-based biopharma sponsors; high trial site density).
Europe: strong R&D hubs (Denmark — LEO; Switzerland; UK) and active regulatory pathways for trials.
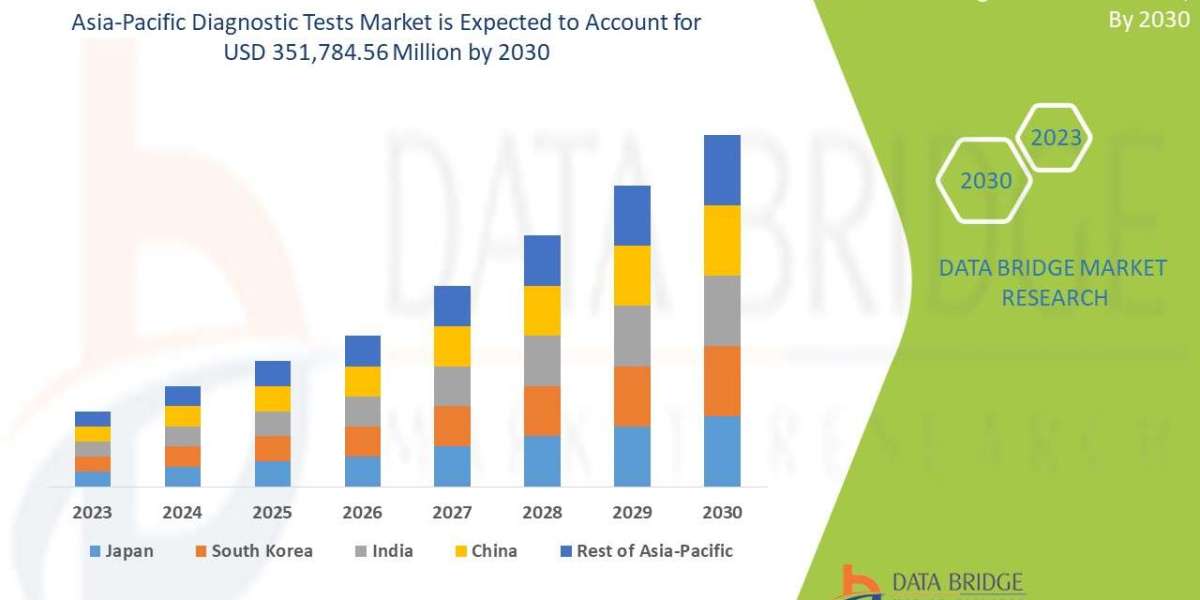
Asia-Pacific: growing patient recruitment sites (cost advantages & increasing trial sites), important for global Phase 3 enrollment. (Market reports highlight APAC expansion).
Emerging Trends
Oral small molecules with improved safety profiles (continued JAK research; protein degraders) entering clinics.
Pediatric label expansion — trials now include younger children (tapinarof, dupilumab extension programs).
Topical next-gen molecules (e.g., tapinarof) with favorable tolerability moving through registration.
Top Use Cases (clinical-trial endpoints / indications sponsors focus on)
Moderate-to-severe AD in adults (primary registrational population).
Pediatric AD (label expansion and safety in younger cohorts).
Maintenance of remission / long-term safety / steroid-sparing regimens. (Common endpoints in extension trials.)
Major Challenges
Comparator and clinical-meaningful endpoint expectations — trials must show clinically meaningful benefits beyond established biologics.
Safety signal management for systemics (JAK class) — regulators and payers scrutinize long-term risk.
High cost & long timelines to gather registration-grade efficacy + safety evidence (especially pediatric/long-term).
Attractive Opportunities
Oral, safe, best-in-class alternatives to injectables (if achieved) could capture substantial share — big commercial prize.
Topicals for paediatric or limited-area disease — faster routes to market with lower systemic risk.
Biomarker-driven, personalized therapies (targeting specific immune pathways) to differentiate from broad immunomodulators. (Implied by novel modalities in early trials.)
Key factors of market expansion
Clinical success vs Dupixent (efficacy, safety, convenience) — positive readouts drive more trials and investment.
Growth in pediatric indications — enlarges addressable population.
Emergence of new modalities (protein degraders, next-gen small molecules) attracting capital and trial activity.
Geographic expansion of trial sites (APAC / LATAM) — reduces recruitment time/costs for global Phase 3.
Quick recommendations (if you want follow-up work)
I can produce a table listing ~15 leading sponsors with: (a) number of registered AD trials (ClinicalTrials.gov count), (b) flagship AD candidate(s), and (c) most recent trial phase/readout and a citation for each — helpful for competitive landscaping.
Or I can make a one-page PPT summarizing the above for stakeholders.